Abstract
Due to their biological and clinical features, acute myeloid leukemia (AML) with Nucleophosmin-1 (NPM1) gene mutations represents a distinct entity within the WHO classification 2022. As per ELN classification, they are mainly categorized either as favorable or intermediate-risk disease, and as such patients are referred for allogeneic hematopoietic stem cell transplantation (allo-HSCT) at disease relapse, during CR1 or, less frequently, due to primary refractoriness. However, even when transplanted, relapse remains the most common cause of treatment failure. Besides conditioning intensity, presence of measurable residual disease (MRD) at time of allo-HSCT has recently been shown to be associated with an increased risk of relapse post-transplant. Yet, the reported effect might vary according to genetic and immunophenotypic diversity of the disease, transplant setting and MRD technology used.
Here, we aim to determine the impact of NPM1 presence assessed via qPCR on post allo-HSCT outcomes.
This retrospective study included adult AML patients harboring an NPM1 mutation (NPM1mut) who received a first allo-HSCT between January 2011 and January 2022 at four German transplant centers. NPM1 mutation status was assessed at diagnosis, and monitored throughout the treatment course by qPCR. NPM1 MRD monitoring was performed as previously described (Papadaki et al., 2009, sensitivity of 10−6). MRD status pre-transplant was correlated with outcome. There were no restrictions regarding donor type or conditioning intensity
We identified 161 adult AML patients with available pre-transplant MRD status. Median age of the entire cohort was 55 years (21-75). The majority of patients (86%) had cytogenetically normal AML and presented in hematologic remission (CR1, CR2) (69%) at time of allo-HSCT. Whereas NPM1mut was measurable in all patients with active disease, 85/111 (77%) patients in remission had either NPM1mut persistence after antecedent induction/consolidation therapy (n=68) or molecular relapse (n=17). Conditioning intensity used was RIC in 57, MAC in 12, sequential-RIC in 88 and sequential-MAC in 4 patients. Most patients (n=90) were transplanted from matched unrelated donor (matched related: n=37, haploidentical: n=34), using preferentially PBSCs as graft source (n=146).
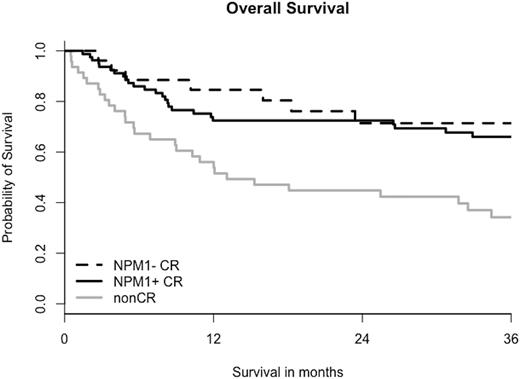
At a median follow-up of 28 months (3-127), the estimated probability of OS and LFS at 2 years was 63% and 55%, respectively. When patients are stratified into groups with active disease, vs NPM1+CR vs NPM1- CR, 2-year OS and LFS show a significant difference (45% vs. 73% vs. 71%; p=0.002 and 55% vs. 62% vs. 67% p=0.005, respectively). Yet, if considering only patients in remission, no survival difference according to NPM1-MRD status at transplantation was detected (OS, p=0.49 and LFS, p=0.33). Likewise, cumulative incidence of relapse at 2 years was 44%, 15% and 17% (p=0.001); however among remission patients there was no significant difference between MRD+ and MRD- patients before allo-HSCT (p=0.96). CI of NRM at 1-year was 14%, 18% and 8% for non-CR, NPM1+ CR and NPM1- CR patients (p=0.9).
Intriguingly, MRD positivity as assessed per qPCR at time of transplant did not impact on post-transplant outcome in our cohort of NPM1 positive AML.
Disclosures
Metzeler:Novartis: Consultancy; Abbvie: Consultancy; Celgene: Consultancy; Curis Inc: Research Funding; Jazz: Consultancy; Pfizer: Consultancy. Schmid:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Research Funding; Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal